In a latest examine printed within the journal Nature Metabolism, researchers investigated the security and efficacy of AMG 133 (maridebart cafraglutide), an engineered molecule, for weight reduction. They discovered that AMG 133 demonstrated weight reduction in cell-based techniques and animal fashions whereas bettering metabolic markers. Additional, in a section 1 medical trial in overweight individuals, AMG 133 confirmed an appropriate security profile and important, dose-dependent weight reduction.
 Examine: A GIPR antagonist conjugated to GLP-1 analogues promotes weight reduction with improved metabolic parameters in preclinical and section 1 settings. Picture Credit score: White bear studio / Shutterstock
Examine: A GIPR antagonist conjugated to GLP-1 analogues promotes weight reduction with improved metabolic parameters in preclinical and section 1 settings. Picture Credit score: White bear studio / Shutterstock
Background
Weight problems, a pervasive public well being problem, calls for efficient and secure therapeutics. Incretin-based remedies, notably glucagon-like peptide 1 receptor (GLP-1R) agonists, have demonstrated important weight discount and cardiovascular advantages. Nevertheless, the demand persists for weight problems remedies with enhanced efficacy, much less frequent dosing, and improved tolerability. To handle this want, researchers explored using multi-specific agonist peptides in opposition to GLP-1 and gastric inhibitory peptide (GIP) pathways.
Genome-wide research supported the contribution of the GIP receptor (GIPR) locus to physique weight regulation. Bispecific molecules engineered by combining GIPR antagonism with GLP-1R agonism confirmed promising preclinical outcomes, inducing weight reduction and metabolic enhancements in overweight mice and monkeys. AMG 133 is one such bispecific molecule developed by conjugating a human monoclonal GIPR-antibody with two GLP-1 analog agonist peptides. Researchers within the current examine investigated the security, pharmacological properties, and efficacy of AMG 133 in preclinical and medical settings.
Concerning the examine
Within the current examine, AMG 133 was synthesized as an antibody-peptide conjugate utilizing an amino acid linker, and its pharmacokinetic (PK) properties have been characterised in vitro. The cell-based useful assays concerned using recombinant human embryonic kidney (HEK) 293T cells expressing human or cynomolgus monkey GIPR and Chinese language hamster ovary (CHO) cells expressing rat or mouse GIPR. Cyclic adenosine monophosphate (cAMP) accumulation was measured. The PK properties of intact and whole AMG 133 have been evaluated by injecting the molecule in mice and overweight feminine cynomolgus monkeys.
Within the medical phase of the examine, a section 1, randomized, placebo-controlled, double-blind trial was performed to guage the tolerability, security, PK, and pharmacodynamics (PD) of single ascending doses (SADs) and a number of ascending doses (MADs) of AMG 133 in adults with weight problems. Whereas the first endpoints have been security and tolerability, the secondary endpoints have been PK and immunogenicity. Additional, PD biomarkers (together with weight) have been thought-about as exploratory endpoints.
In seven SAD cohorts, 49 overweight individuals have been enrolled and randomized to obtain AMG 133 (21–840 mg) or placebo for as much as 150 days. The imply age of those individuals was 45.5 to 53.8 years and their physique mass index (BMI) was 32.5 to 34.8 kg m−2 In three MAD cohorts, 26 overweight individuals have been enrolled and randomized to obtain AMG 133 (140, 280 or 420 mg) or placebo for as much as 207 days. The imply age of those individuals was 40.3 to 51.6 years and their BMI was 32.5 to 34.2 kg m−2. Not one of the individuals had a historical past of diabetes mellitus.
Outcomes and dialogue
The molecular weight of AMG 133 was discovered to be 153,514 Da. Within the mobile assays, AMG 133 confirmed antagonist exercise in opposition to human, cynomolgus monkey, and rat GIPR. The AMG 133 murine surrogate may scale back meals consumption and blood glucose ranges and induce weight reduction in mice. Dose-dependent enhancements in blood glucose, plasma insulin, and lipid ranges have been additionally noticed. AMG 133 therapy in overweight monkeys resulted in a discount in physique weight, whole power consumption, fasting triglycerides, insulin, and ldl cholesterol after six weeks.
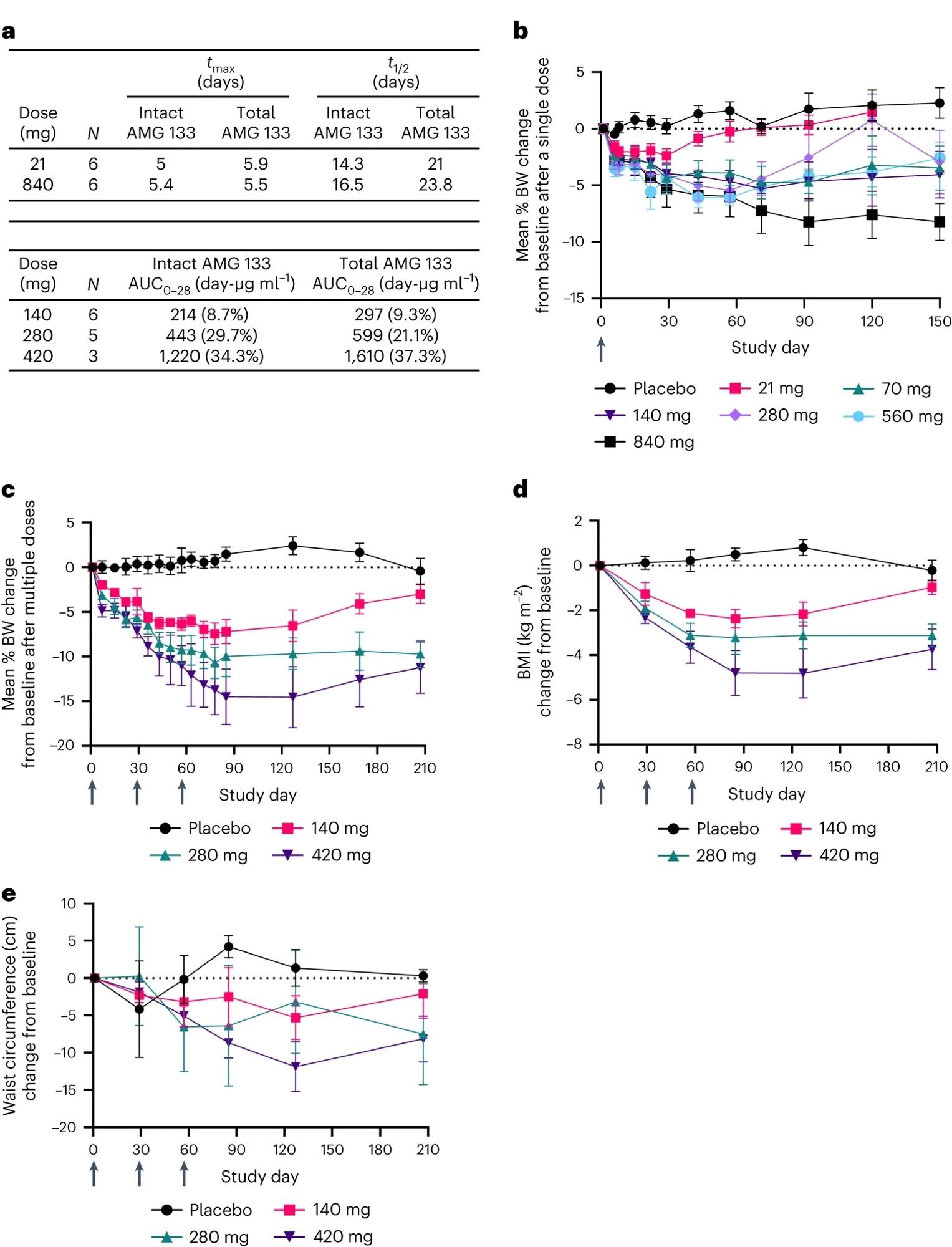 a, AMG 133 PK parameters; tmax is introduced because the median and t1/2 is introduced because the geometric imply. AUC0–28 is introduced because the geometric imply (CV%) after the final dose of AMG 133 on day 57 for MAD cohorts. b–e, Imply (s.e.m.) p.c change from baseline in BW after single doses, n = 6–7 for AMG 133 and n = 12 for placebo at day 1 (b) and a number of doses, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (c). Imply (s.e.m.) change from baseline in BMI, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (d) and waist circumference, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (e) after a number of doses of AMG 133. Arrows point out when the investigational product was administered: at day 1 within the SAD cohorts (b) and at day 1, 29 and 57 within the MAD cohorts (c–e).
a, AMG 133 PK parameters; tmax is introduced because the median and t1/2 is introduced because the geometric imply. AUC0–28 is introduced because the geometric imply (CV%) after the final dose of AMG 133 on day 57 for MAD cohorts. b–e, Imply (s.e.m.) p.c change from baseline in BW after single doses, n = 6–7 for AMG 133 and n = 12 for placebo at day 1 (b) and a number of doses, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (c). Imply (s.e.m.) change from baseline in BMI, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (d) and waist circumference, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (e) after a number of doses of AMG 133. Arrows point out when the investigational product was administered: at day 1 within the SAD cohorts (b) and at day 1, 29 and 57 within the MAD cohorts (c–e).
Within the section 1 medical examine, AMG 133 confirmed an appropriate security and tolerability profile. Medical security laboratory parameters (electrolytes, kidney perform, and hematology) and echocardiogram parameters confirmed no important variations between the therapy teams. No extreme or critical antagonistic occasions (AEs) have been reported. The widespread AEs have been delicate gastrointestinal signs, predominantly nausea and vomiting, which usually resolved inside 48 hours. Though a discount was noticed in fasting glucose ranges, no hypoglycemia-related occasions have been reported.
Moreover, no clinically important adjustments in blood stress have been noticed with AMG 133, and coronary heart fee will increase throughout the regular vary have been famous. AMG 133 therapy led to elevated free fatty acids, notably within the 420 mg group. Transient decreases in whole ldl cholesterol, low-density lipoprotein, and triglycerides have been noticed throughout teams (together with placebo).
AMG 133 confirmed a dose-proportional enhance with most plasma concentrations attained round 4 to 7 days post-dose within the SAD cohort and after 4 to six days within the MAD cohort. The imply half-life for intact AMG 133 ranged from 14 to 16 days, and that for whole AMG 133 ranged from 21 to 24 days.
Importantly, AMG 133 therapy was discovered to decrease the imply physique weight, BMI, and waist circumference from baseline in a dose-dependent method within the individuals.
Conclusion
In conclusion, this examine’s findings counsel that AMG 133 could also be a probably viable therapeutic choice for weight administration, given its favorable security profile, prolonged half-life, and substantial and sustained weight reduction. Additional analysis in a section 2 medical trial setting is warranted to verify these findings.
Journal reference:
- A GIPR antagonist conjugated to GLP-1 analogues promotes weight reduction with improved metabolic parameters in preclinical and section 1 settings. Véniant, M.M. et al., Nature Metabolism (2024), DOI: 10.1038/s42255-023-00966-w, https://www.nature.com/articles/s42255-023-00966-w

